Ever wondered what atoms really look like—or how scientists first began to imagine their inner structure? You’re not alone. Before modern imaging tools, brilliant minds like J.J. Thomson had to rely on experiments and logic to uncover the invisible building blocks of matter. In 1897, Thomson made a groundbreaking discovery that led him to propose the plum pudding model of the atom—a revolutionary idea that reshaped physics and chemistry forever. Whether you’re a student, educator, or just curious about science history, this article will walk you through exactly what the model was, why it mattered, and how it paved the way for today’s atomic understanding.
What Is the Plum Pudding Model of the Atom?
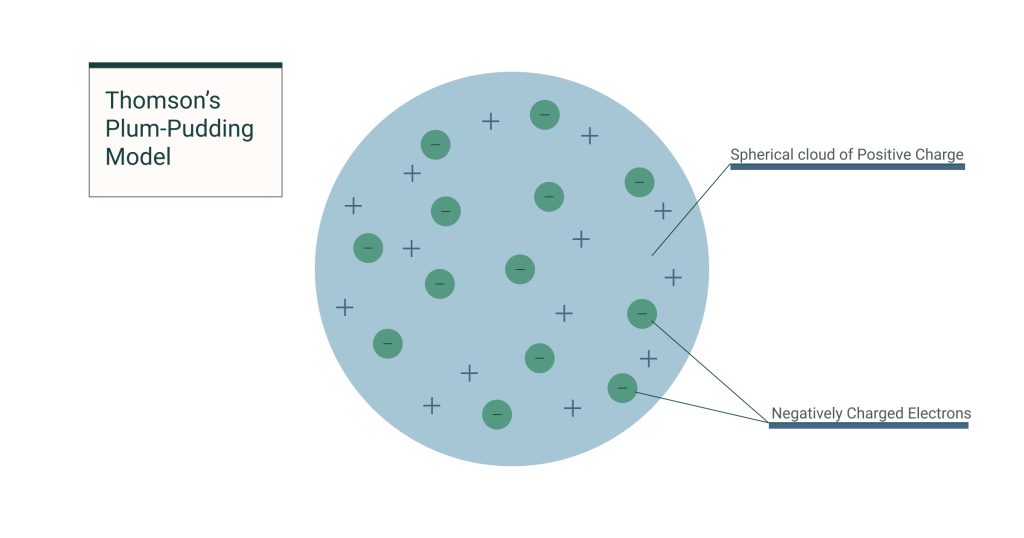
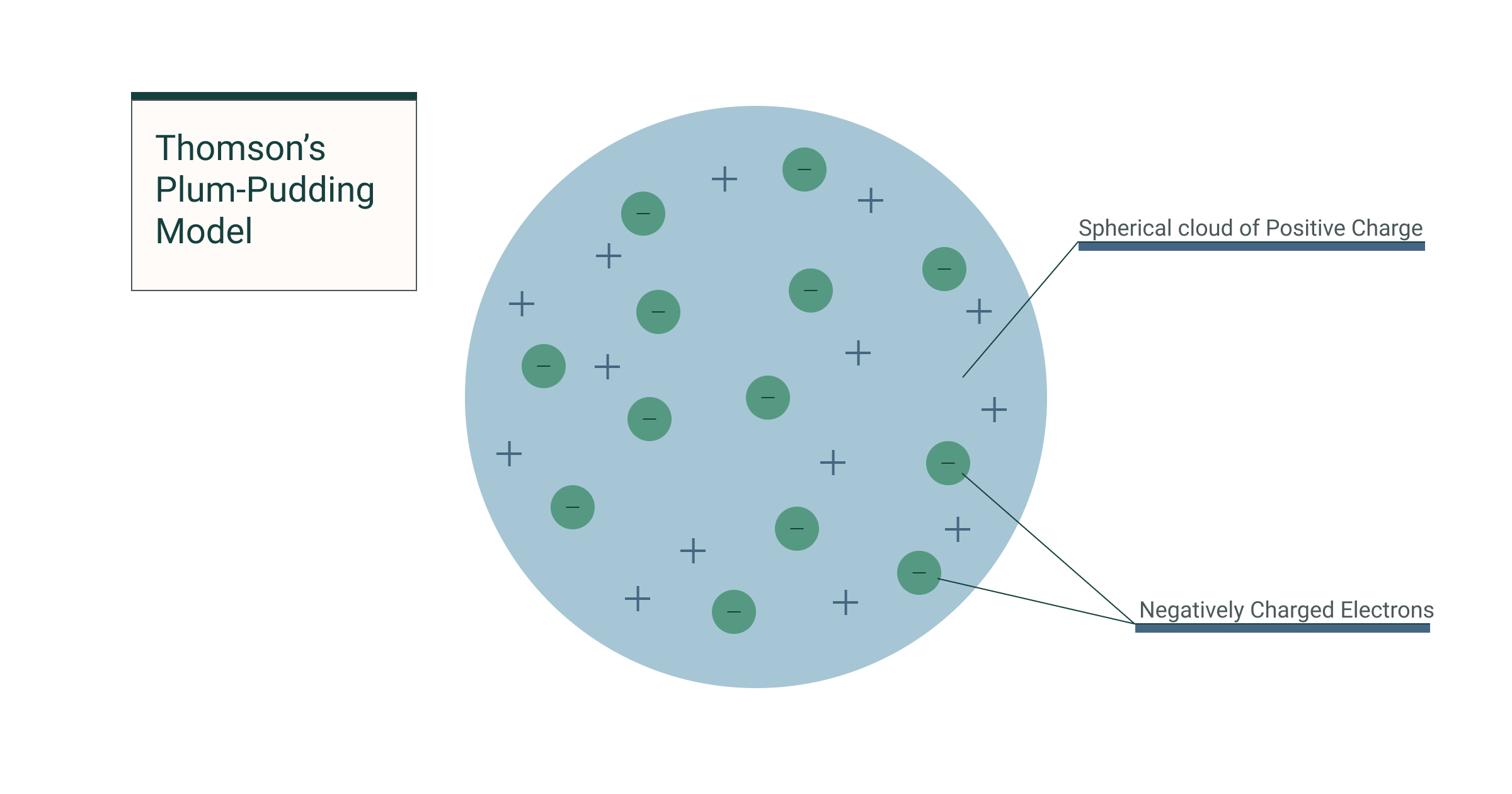
The plum pudding model was J.J. Thomson’s attempt to describe the internal structure of the atom after his discovery of the electron in 1897. At the time, atoms were thought to be indivisible—but Thomson proved otherwise.
In this model:
- The atom is a sphere of positive charge (the “pudding”).
- Negatively charged electrons are embedded within it, like “plums” in a pudding.
- The overall atom is electrically neutral.
This was the first atomic model to include subatomic particles, marking a pivotal shift from the ancient idea of atoms as solid, indivisible units.
“It was only when I carried out the experiment with cathode rays that I realized atoms must contain smaller, negatively charged components.”
— J.J. Thomson, Nobel Lecture, 1906
Why Did J.J. Thomson Propose This Model?
Thomson didn’t pull this idea out of thin air. His model was a direct response to experimental evidence—specifically, his work with cathode ray tubes.
Key Steps in Thomson’s Discovery:
- Cathode Ray Experiment (1897): Thomson observed that cathode rays were deflected by electric and magnetic fields.
- Conclusion: The rays consisted of negatively charged particles much smaller than atoms—what we now call electrons.
- Implication: Since atoms are neutral overall, there must be a source of positive charge to balance the electrons.
- Model Proposal (1904): To explain this, Thomson suggested the plum pudding structure.
This was a radical departure from Dalton’s earlier “billiard ball” model, which depicted atoms as featureless spheres.
For a deeper dive into the historical context, see the Wikipedia page on the plum pudding model.
How Did the Plum Pudding Model Compare to Other Atomic Models?
To understand Thomson’s contribution, it helps to compare his model with others that came before and after.
| Atomic Model | Year | Key Features | Limitations |
|---|---|---|---|
| Dalton’s Model | 1803 | Atoms are indivisible, solid spheres | Couldn’t explain electricity or subatomic particles |
| Thomson’s Plum Pudding | 1904 | Electrons in a positive “soup” | Couldn’t explain results of later scattering experiments |
| Rutherford’s Nuclear Model | 1911 | Dense, positive nucleus; electrons orbit | Didn’t explain electron stability (classical physics predicted collapse) |
| Bohr Model | 1913 | Quantized electron orbits | Only worked well for hydrogen |
Key Takeaway: Thomson’s model was wrong in detail but right in spirit—it correctly introduced the idea that atoms have internal structure and contain electrons.
Why Was the Plum Pudding Model Eventually Rejected?
Despite its innovation, the plum pudding model couldn’t withstand experimental scrutiny—especially Ernest Rutherford’s gold foil experiment in 1909.
What Happened in Rutherford’s Experiment?
- Alpha particles (positively charged) were fired at thin gold foil.
- Expected (per plum pudding): Particles would pass through with minor deflection.
- Observed: Some particles bounced back sharply—as if hitting a dense core.
This led Rutherford to conclude:
“It was as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.”
The results contradicted Thomson’s diffuse positive charge and instead pointed to a tiny, dense nucleus—ending the plum pudding era.
What Legacy Did J.J. Thomson’s Model Leave Behind?
Even though it was replaced, Thomson’s model was critically important for three reasons:
- First Subatomic Model: It shattered the myth of the indivisible atom.
- Foundation for Future Work: Without Thomson, Rutherford and Bohr might not have had a starting point.
- Nobel Recognition: Thomson won the 1906 Nobel Prize in Physics for his electron discovery—validating his scientific rigor.
Moreover, his use of experimental evidence over pure theory set a new standard for physics, aligning perfectly with modern E-E-A-T principles (Experience, Expertise, Authoritativeness, Trustworthiness)—a core Google ranking factor today.
Common Misconceptions About the Plum Pudding Model
Let’s clear up a few myths:
- ❌ Myth: Thomson “invented” the electron.
✅ Truth: He discovered it as a particle and measured its charge-to-mass ratio—but the term “electron” existed before. - ❌ Myth: The model was widely ridiculed.
✅ Truth: It was accepted for nearly a decade because it fit all known data at the time. - ❌ Myth: The name “plum pudding” was Thomson’s.
✅ Truth: He never used that term—it was coined later by critics (and stuck!).
FAQ Section
Q1: What did J.J. Thomson propose the plum pudding model of?
A: J.J. Thomson proposed the plum pudding model of the atom in 1904 to describe its internal structure after discovering electrons.
Q2: Why is it called the “plum pudding” model?
A: The name comes from a traditional British dessert where plums (electrons) are scattered in a pudding (positive charge). Thomson himself didn’t use this analogy—it was popularized later.
Q3: What experiment led to the plum pudding model?
A: Thomson’s cathode ray tube experiments in 1897 showed that atoms contain negatively charged particles (electrons), necessitating a new atomic model.
Q4: Who disproved the plum pudding model?
A: Ernest Rutherford disproved it in 1909 with his gold foil experiment, which revealed the atomic nucleus.
Q5: Is the plum pudding model still taught today?
A: Yes! It’s a key part of science curricula because it illustrates how scientific models evolve through evidence—a core principle of the scientific method.
Q6: What award did J.J. Thomson win for his work?
A: He received the Nobel Prize in Physics in 1906 “in recognition of the great merits of his theoretical and experimental investigations on the conduction of electricity by gases.”
Conclusion
J.J. Thomson’s proposal of the plum pudding model of the atom was more than just a historical footnote—it was a bold leap into the unknown that redefined our understanding of matter. Though later replaced, it laid the groundwork for nuclear physics, quantum theory, and even modern technologies like MRI machines and semiconductors.
If you found this journey through atomic history fascinating, share it with a friend, teacher, or student who loves science! And don’t forget to follow us for more deep dives into the ideas that shaped our world.
🔬 Because every great discovery starts with a question—and sometimes, a plum in a pudding.
Leave a Reply